News and Publications
Systematic review of outcomes reported in clinical research on nephronophthisis: how do they align with SONG Kids priorities?
Dahmer-Heath, Optenhövel, Hechler et al, Pediatric Nephrology, August 2025. https://doi.org/10.1007/s00467-025-06912-0
Background
Nephronophthisis (NPH) is a very rare inherited kidney disease in children. Its symptoms, as well as the genes that cause it, can vary greatly from child to child. NPH is responsible for about 1 in 10 cases of kidney failure in children. At the moment, there is no cure and no treatment that stops or slows down the disease. To make progress toward future therapies, it is important not only to study the kidneys themselves, but also to listen to what matters most to patients and families. These insights, known as patient-reported outcome measures (PROMs), ensure that research and treatments address the aspects of the disease that truly affect everyday lif
Objectives
We wanted to find out which types of outcomes have been studied in NPH so far: medical data from doctors, laboratory or imaging results (so-called “surrogate parameters”), and patient-reported outcomes. We then compared these findings with the outcomes identified by the international SONG (Standardized Outcomes in Nephrology) project, which highlights the measures most important to children living with kidney disease
Data sources
We searched the medical database MEDLINE to identify all published studies on NPH
Study eligibility criteria
We included original studies published after 1988, written in English, that reported outcomes in at least four patients with NPH and focused on people rather than laboratory research
Results
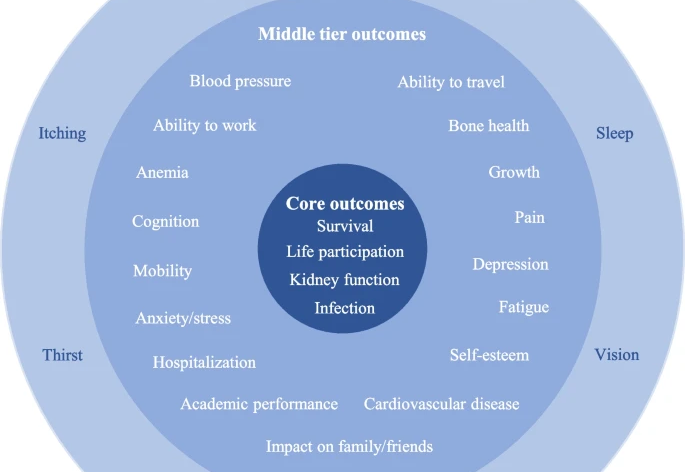
From over 1000 publications screened, 90 studies met our inclusion criteria. All of these studies reported medical outcomes, and most also included laboratory or imaging findings. However, less than half (41%) looked at outcomes reported directly by patients and families. When we compared the results with the SONG project, we found only a partial overlap. Only 20 studies reported more than one of the core SONG outcomes. Importantly, none of the studies used questionnaires or tools specifically designed and validated for children with NPH.
Limitations
Most studies focused on genes and biology, not on the patient’s lived experience. Almost all studies looked back at existing patient files rather than following children over time. We also could not tell whether outcomes came directly from children or only from their parents, which is an important difference for future research
Conclusions and implications of key findings
Research in NPH has mainly focused on medical and laboratory data, with little attention given to the experiences of children and families. This means that important aspects of living with NPH — such as daily activities, school participation, friendships, and overall quality of life — are often overlooked in studies. To address this gap, there is a clear need for new, disease-specific tools that capture patient-reported outcomes in NPH. Such tools will help ensure that future treatments and clinical trials focus on what truly matters to patients and families. Developing one of these tools is a key goal of the ongoing work within the TheRaCil European consortium.
Background
Nephronophthisis (NPH) is a rare hereditary cystic kidney disease, characterized by a highly variable clinical and genetic presentation, accounting for up to 10% of kidney failure in children. Despite advances in understanding its molecular basis and phenotypic spectrum, no causative therapies exist, and clinical trials remain absent. To support future treatment development, patient-reported outcome measures (PROMs) tailored to NPH should be defined to prioritize outcomes meaningful to patients and families.
Objectives
This study aimed to analyze the use of clinical data, surrogate parameters, and patient-reported outcomes in NPH research to date, with a focus on the Standardized Outcomes in Nephrology (SONG) project outcomes validated for children with chronic kidney disease (SONG Kids).
Data sources
A systematic search of the MEDLINE database was conducted for NPH studies.
Study eligibility criteria
Studies published after 1988, written in English, reporting at least one human clinical outcome, with a sample size of n ≥ 4, and using original data were considered eligible.
Results
A total of 1066 records were retrieved through the search, of which 821 full-text reports were assessed for eligibility. Of these, 90 studies met eligibility criteria and were included in the review. While 100% of the studies reported clinical outcomes and 85% included surrogate parameter, only 41% examined patient-reported outcomes. Overlap between the SONG Kids outcome set and the outcomes identified in this study was moderate. Only 20 studies reported more than one SONG core outcome, while 24% and 66% of studies reported at least one middle tier or outer tier outcome, respectively. None of these studies used instruments validated for NPH.
Limitations
The majority of studies focused primarily on molecular and genetic aspects, with clinical outcomes addressed only as a secondary consideration. The review incorporated only one prospective study, while the remaining studies were retrospective in nature. Differentiation between outcomes reported by children and those reported by parents was not possible in the included studies; this important distinction must be taken into account in the development of future PROMs for NPH.
Conclusions and implications of key findings
Studies in NPH addressed both clinical outcomes and surrogate parameters, but there is a notable absence of measures related to life participation and patient-reported outcomes. Disease group-specific instruments fall short in adequately reflecting the symptoms of individual diseases, emphasizing the necessity for the development of disease-specific PROMs for NPH.
Open Science Framework (OSF) registration: https://doi.org/10.17605/OSF.IO/658BR