News and Publications
KIF13B controls ciliary protein content by promoting endocytic retrieval and suppressing release of large extracellular vesicles from cilia
Rezi et al, Current Biology, October 2025. https://doi.org/10.1016/j.cub.2025.08.022
Understanding How Kidney Cells Control Cilia Function: The Role of KIF13B
Background
Cilia are tiny, hair-like structures on the surface of many cells, including kidney cells. They act as “antennae,” helping cells sense their surroundings and communicate signals that keep tissues healthy. When cilia do not work properly, a group of rare diseases called ciliopathies can arise, which often affect the kidneys. To stay healthy, cilia must constantly renew and adjust the proteins they contain — removing old or damaged ones and bringing in new ones.
What this study found
This study focused on a motor protein called KIF13B, which helps move materials inside cells. The researchers discovered that KIF13B plays an important role in controlling which proteins stay inside the cilia and which ones are removed. They studied kidney cells grown in the laboratory and compared normal cells with cells lacking the KIF13B protein.
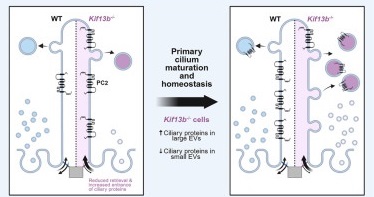
In cells missing KIF13B, important ciliary proteins such as polycystin-2 (PC2) — a protein linked to kidney cyst formation — built up abnormally inside the cilia. These cells also released more and larger extracellular vesicles (EVs) — tiny membrane bags that cells use to send materials out — containing unusual proteins not typically found there. Over time, the lack of KIF13B led to major changes in how proteins were sent in and out of the cilia, suggesting that KIF13B helps keep this balance under control. Studying this mechanism also revealed several proteins that were not previously found in cilia, such as CCDC198 and ZDHHC5.
Why this matters
By identifying how KIF13B regulate what enters and leaves the cilia, the study provides new clues about how cilia maintain their normal composition and function. Because cilia defects cause kidney and other organ problems in ciliopathies, understanding these processes may help researchers find new candidate genes involved in these diseases and eventually guide new treatment strategies.
Take-home message
This research reveals that KIF13B helps kidney cells manage the flow of proteins in and out of their cilia — a process essential for healthy kidney function. Disruption of this control can lead to ciliary dysfunction, one of the key problems in ciliopathies. The proteins CCDC198 and ZDHHC5, newly shown in this study to localize to cilia, may represent new leads for understanding and diagnosing pediatric renal ciliopathies in the future.
Dynamic control of ciliary membrane protein content is crucial for the organelle’s homeostasis and signaling function and involves removal of ciliary components by intraflagellar transport (IFT) and BBSome-mediated export, endocytic retrieval, and/or extracellular vesicle (EV) shedding. We report that the kinesin-3 motor KIF13B regulates ciliary protein composition and EV shedding in cultured kidney epithelial cells, with effects that vary over time. In early stages of ciliation, Kif13b-/- cells aberrantly accumulate polycystin-2 (PC2) within cilia and release large EVs enriched with CCDC198 and the centriole distal appendage protein CCDC92, which also localizes to the ciliary tip. These cells also produce fewer small EVs through the neutral sphingomyelinase 2 pathway. Upon cilia maturation, Kif13b-/- cells accelerate large EV release of numerous ciliary proteins, including PC2, BBSome, and IFT components, which correlates with gradual depletion of CCDC92 and PC2 from the ciliary tip and shaft, respectively. Furthermore, over time, Kif13b-/- cells show an upregulation in the release of small EVs, which differ in composition from wild-type small EVs. Specifically, mutant small EVs lack several proteins that are enriched in small EVs from BBSome-deficient cells, including palmitoyl transferase ZDHHC5, which localizes to cilia where it accumulates upon BBSome dysfunction and regulates ciliary length and PC2 levels. Our results suggest that KIF13B acts at the level of centriole distal appendages to limit ciliary protein entry and promote endocytic retrieval downstream of the BBSome, thereby suppressing EV release from cilia. Furthermore, the ciliary localization of CCDC198 and ZDHHC5 indicates they are potential novel ciliopathy candidates.