News and Publications
Urinary peptide signature distinguishes autosomal recessive polycystic kidney disease from other causes of chronic kidney disease
Burgmaier et al, Clinical Kidney Journal, Volume 18, Issue 5, sfaf093, May 2025. https://doi.org/10.1093/ckj/sfaf093
Background
Diagnosing autosomal recessive polycystic kidney disease (ARPKD) in children can be difficult because the symptoms can vary widely and sometimes look similar to other types of chronic kidney disease (CKD). In this study, researchers aimed to find a way to better distinguish ARPKD from other kidney conditions by looking at molecules found in urine.
Methods
The researchers analyzed urine samples from three groups:
- 58 children with ARPKD
- 662 children with other forms of CKD
- 45 healthy children
They used a technique that separates and identifies small protein fragments in urine (called the urinary peptidome) to search for patterns that are unique to ARPKD.
Results
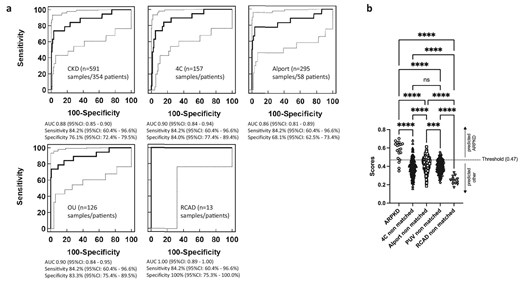
They discovered a unique pattern made up of 77 tiny protein fragments (called peptides) that was specific to children with ARPKD. When tested on a new set of patients, this peptide pattern correctly identified ARPKD in 84% of cases and correctly ruled it out in 100% of non-ARPKD cases. Even when tested in a broader group of children with various kidney diseases, the test still performed well. Most of the peptides in the pattern came from collagen, suggesting damage to the structure of the kidney. A few others were related to calcium-binding proteins, which may provide new clues about how ARPKD affects the body.
Conclusions
This study has found a promising new way to help diagnose ARPKD by analyzing urine samples. The specific pattern of protein fragments could help doctors more accurately tell ARPKD apart from other kidney diseases. It may also help researchers better understand how ARPKD affects the kidneys, opening up new directions for future research and treatments.
Background
The diagnosis of autosomal recessive polycystic kidney disease (ARPKD) can be hampered by its pronounced phenotypic variability and ARPKD-mimicking phenocopies. Here, for the first time we specifically studied the urinary peptidome of patients with ARPKD with the aim of distinguishing ARPKD from other causes of chronic kidney disease (CKD).
Methods
Fifty-eight urine samples from patients with ARPKD, 662 urine samples from paediatric patients with CKD with various other CKD aetiologies and 45 samples from healthy children were included. The urinary peptidome was analysed by capillary electrophoresis/mass spectrometry.
Results
A 77-peptide signature specific for ARPKD was identified. Application of this signature in a matched random validation set of 19 samples of patients with ARPKD, 23 samples from patients with other CKD and 21 samples from healthy individuals led to a sensitivity of 84.2% [95% confidence interval (CI) 60.4–96.6], a specificity of 100% (95% CI 92.0–100%) and an area under the receiver operating characteristics curve (AUC) of 0.994 (95% CI 0.93–1.00). The 77-peptide signature displayed a specificity of 76.1% (95% CI 72.4–79.5) and an AUC of 0.88 (95% CI 0.85–0.90) in 591 samples from non-matched children with various CKD aetiologies. The signature was primarily (83%) composed of collagen fragments indicating structural damage. Of the remaining peptides, five originated from proteins known to bind to calcium potentially linking the current work to defaults in calcium signalling in polycystic disease.
Conclusions
We determined a urinary peptide signature that identifies paediatric patients with ARPKD with high precision among a population of children with CKD. Knowledge of the identity of the underlying peptides offers a novel starting point for discussion of possible pathophysiological processes involved in ARPKD.